Introduction
Indocyanine Green (ICG) has emerged as a pivotal tool in modern surgical oncology, significantly enhancing the visualization and management of various cancer types. Originally introduced in the 1950s for assessing liver function, ICG's applications have expanded dramatically, particularly within the realm of oncological surgery. With its ability to provide real-time imaging of tumor margins and lymphatic drainage, ICG has transformed the approach to cancer surgery, promoting better surgical outcomes and minimizing patient morbidity. This article delves into the utilization of ICG across diverse cancer surgical specialties, emphasizing its role in improving surgical efficacy and patient care while exploring emerging trends and future prospects.
Understanding Indocyanine Green (ICG)
Chemical and Physical Properties-
Indocyanine Green (ICG) is a synthetic dye first approved by the FDA in 1959 for various medical applications. Its unique properties make it particularly suited for surgical applications:
- Water-Solubility: This property allows ICG to be easily administered intravenously.
- Near-Infrared Fluorescence: ICG fluoresces when exposed to near-infrared (NIR) light (wavelengths between 700 and 800 nm), allowing for improved tissue penetration compared to visible light.
- Rapid Elimination: ICG is rapidly absorbed by the liver and eliminated through bile, boasting a half-life of approximately 2-5 minutes in the bloodstream.
- Pharmacokinetics and Administration Protocol:ICG typically requires an intravenous injection for surgical utilization. The recommended dosage varies based on the procedure but often ranges from 0.5 to 1.0 mg/kg body weight. After administration, fluorescence imaging can be performed almost instantaneously, making it an ideal tool for real-time intraoperative assessment.
- ICG is a near-infrared fluorescent dye that, upon administration, binds to plasma proteins. When illuminated with near-infrared light, ICG fluoresces, facilitating the visualization of tissues, blood vessels, and lymphatics with remarkable precision. The near-infrared spectrum allows for deep tissue penetration and minimal background fluorescence, which is particularly advantageous in complex surgical fields.
Applications of ICG in Cancer Surgery:
1. Tumor Margin Assessment: Accurate delineation of tumor margins is critical in ensuring complete excision and reducing the risk of local recurrence. ICG plays a vital role in surgeries for various cancers:
Breast Cancer: The intraoperative application of ICG around the tumor bed allows surgeons to visualize cancerous tissue during lumpectomies or mastectomies. This technique has been associated with a significant reduction in re-excision rates. Surgeons can identify the outer limits of the tumor while preserving surrounding healthy tissues, which is crucial for aesthetic outcomes and functional preservation.
Colorectal Cancer: In rectal cancer surgeries, ICG enhances the ability to visualize tumor outlines and differentiate cancerous from non-cancerous tissue. This clarity aids surgeons during mesorectal excisions and minimizes damage to adjacent structures, contributing to better postoperative recovery.
Sarcoma Surgery: In high-grade sarcomas, where margins may be unclear due to infiltrative growth patterns, ICG can assist in identifying the periphery of the tumor, ensuring that adequate surgical margins are achieved during resection.
2. Enhanced Tumor Visualization: ICG can significantly improve the visualization of tumors, particularly in cases where tumors are small, located near vital structures, or have indistinct margins. By providing real-time imaging, ICG allows for more precise tumor localization and minimizes the risk of leaving residual cancerous tissue behind, thereby improving overall outcomes.
3. Sentinel Lymph Node Mapping: The use of ICG in sentinel lymph node biopsy (SLNB) has transformed the management of various cancers.
Breast Cancer
ICG has found wide application in the identification of sentinel lymph nodes (SLNs) in breast cancer surgery. Traditionally, radioactive tracers and blue dyes were used for SLNB; however, ICG provides a non-radioactive, highly accurate, and safe alternative. The injection of ICG into the peritumoral breast tissue enables real-time visualisation of lymphatic pathways and sentinel lymph nodes (SLNs), leading to improved detection rates and streamlined procedures. As a result, patient outcomes are improved by minimizing unnecessary lymph node dissections and the associated complications, such as lymphedema.
Melanoma
ICG has become a standard agent for SLNB in melanoma cases, with studies demonstrating its efficacy in accurately identifying sentinel nodes. The ability to visualize lymphatic drainage in real-time enhances the surgeon's ability to locate and assess sentinel nodes, which is vital for staging and further treatment planning
Endometrial Cancer
ICG fluorescence imaging is increasingly used for sentinel lymph node mapping in endometrial and cervical cancers. Its high sensitivity minimizes the need for extensive lymphadenectomy, reducing morbidity while preserving diagnostic accuracy. This application is especially beneficial for early-stage disease where lymph node involvement is uncertain, allowing for a targeted surgical approach.
4. Enhanced Visualization in Minimally Invasive Surgery: The application of ICG in laparoscopic and robotic surgeries allows for enhanced visualization:
Laparoscopic Resections: In minimally invasive colorectal, gastric, and esophageal cancer surgeries, ICG enhances the surgeon's ability to visualize tumors and lymphatic systems while minimizing trauma to adjacent tissues. By providing real-time imaging, ICG improves the precision of tumor resections, leading to favorable outcomes.
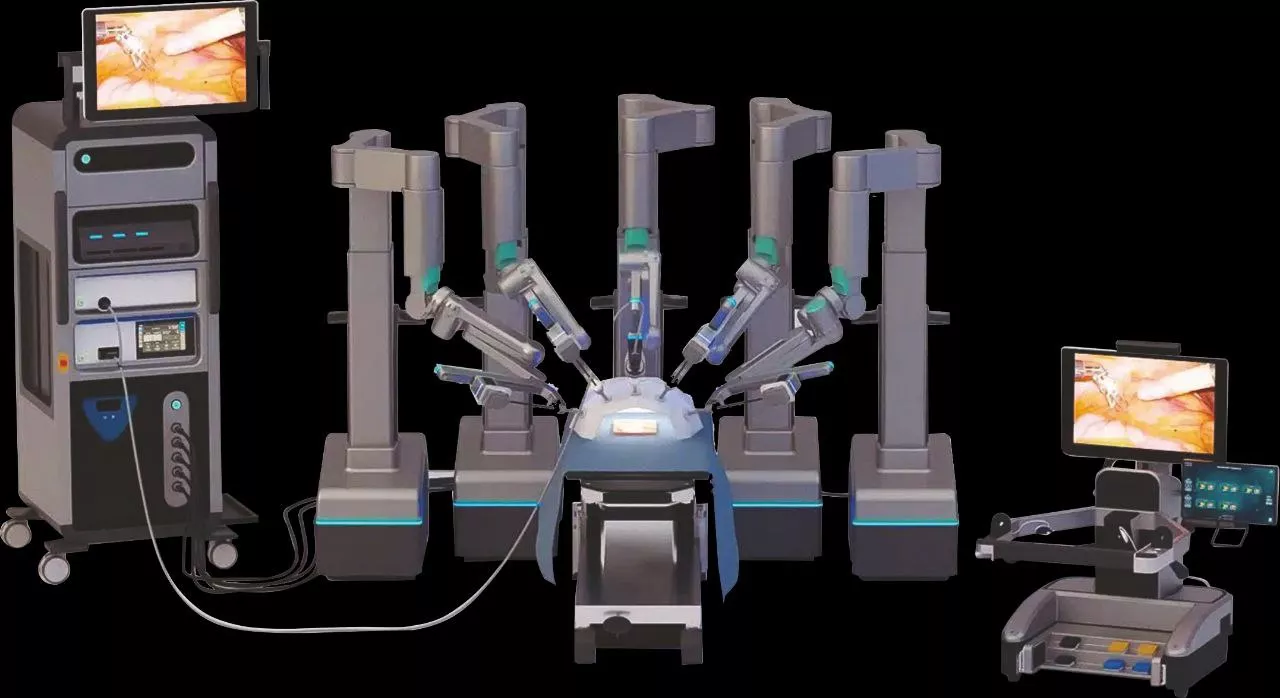
Robotic-Assisted Surgery: In robotic surgery for various cancers, the integration of ICG imaging systems allows surgeons to act with greater precision and confidence. The advantages of real-time imaging combined with robotic platforms enhance surgeons' ability to navigate complex anatomical structures and optimize surgical strategies.
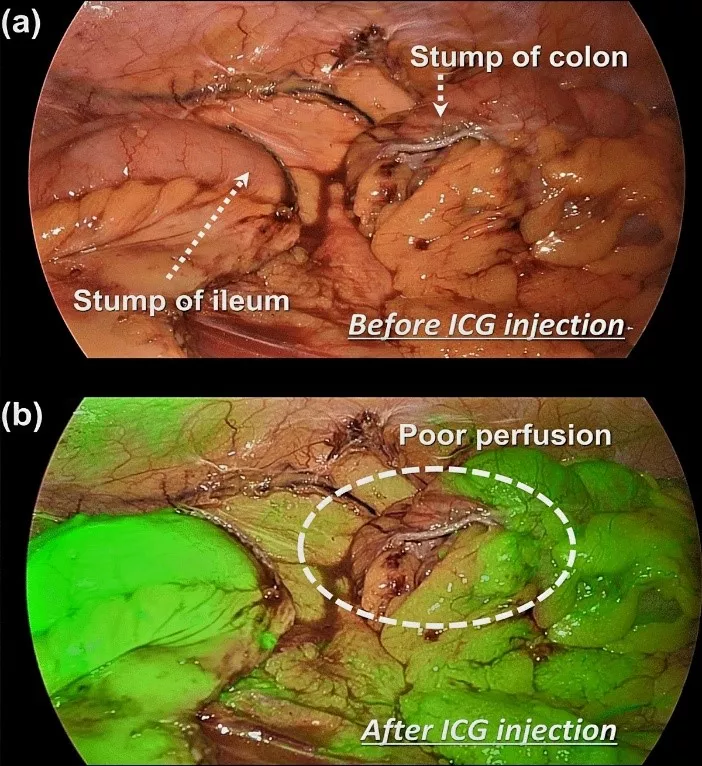
5. Assessment of Bowel Perfusion: Intraoperative Assessment: Surgeons administer ICG to visualize blood flow to the bowel segments. Fluorescence imaging aids in identifying adequately perfused segments ready for connection. Areas with inadequate perfusion will exhibit reduced fluorescence.
Outcomes and Benefits: Surgeons are equipped with the information needed to make informed decisions about bowel sections being anastomosed, significantly reducing postoperative complications such as leaks and ischemia, and helps in faster recovery.
Similar to bowel anastomosis, ensuring robust blood supply during esophago-gastric tube anastomosis during esophagectomy is crucial for surgical success. This real-time perfusion assessment has been shown to reduce rates of anastomotic leaks, which are major complications leading to increased morbidity and prolonged hospital stays.
6. Free Flap and Reconstructive Surgery: ICG has significant applications in reconstructive surgeries, especially for patients undergoing cancer treatments.
In complex reconstructive procedures, such as those needed after oncological resections, ICG is utilized to assess the vascularity of free flaps. By visualizing blood flow to the flap in real time, surgeons can ensure that the tissue is adequately perfused and make immediate adjustments if there are concerns about vascular compromise. This application is particularly important in head and neck reconstructions, as it helps prevent flap failure.
Breast Reconstruction
In immediate or delayed breast reconstruction post-mastectomy, ICG can assist in monitoring the viability of the skin flap and the underlying vascular supply. This can significantly impact the success of the reconstruction and the aesthetic outcomes for the patient.
7. Indocyanine Green (ICG): has emerged as an invaluable and multifaceted tool in various cancer surgeries, extending far beyond its well-established applications in procedures involving breast cancer, colorectal cancer, melanoma, bowel surgeries, and sentinel lymph node evaluations. Initially recognized for its effectiveness in these areas, ICG has demonstrated remarkable versatility and adaptability, becoming a crucial component in a wider array of oncological interventions, including lung cancer surgery. Its real-time imaging capabilities play a critical role in facilitating enhanced visualization of vascular structures and lymphatic drainage, thus providing invaluable assistance in complex resections and organ preservation strategies across multiple oncological domains and procedures.
For instance, in the surgical management of liver cancer, ICG-assisted fluorescence imaging significantly supports the precise identification of tumors and the critical vascular anatomy surrounding the liver. This enhanced visualization not only streamlines the surgical process but also minimizes the risk of post-operative complications, which can be substantial in such intricate procedures where maintaining the integrity of surrounding structures is vital. In lung cancer surgeries, ICG has been effectively utilized to improve the identification of pulmonary nodules and to guide the resection of affected lung tissue. By enhancing the visibility of interstitial vascular networks, surgeons are better equipped to discriminate between cancerous and surrounding healthy lung tissue, thus facilitating more precise resections while preserving lung function.
Furthermore, in the field of head and neck oncology, ICG has proven particularly useful in locating sentinel lymph nodes, thereby improving the accuracy of both staging and treatment planning. Accurate sentinel lymph node mapping is essential for determining the spread of cancer and tailoring subsequent treatments appropriately. The versatility of ICG extends to surgeries involving pancreatectomy and gastric cancer as well. In these complex procedures, ICG enhances the understanding of perfusion dynamics, which is crucial for ensuring that healthy tissue remains viable while removing cancerous tissue. This level of precision and detail in visualization ultimately contributes significantly to better surgical outcomes and improved patient prognoses
Benefits of ICG Utilization
1. *Real-Time Decision-Making:* The real-time feedback provided by ICG allows for immediate adjustments to surgical plans based on intraoperative findings, enhancing the overall quality of care.
2. *Reduced Surgical Morbidity:* With accurate identification of tumor margins and lymphatic pathways, surgeons can minimize the extent of tissue removal, thereby reducing complications associated with larger resections.
3. *Customized Surgical Training:* ICG technology offers educational opportunities for surgical trainees, allowing them to visualize and comprehend anatomical relationships more clearly during procedures and improving their overall surgical skills.
4. *Enhanced Precision:* The ability to distinguish between cancerous and non-cancerous tissues improves the precision of resections, leading to better oncological outcomes. By limiting damage to surrounding structures and identifying critical anatomy, ICG reduces the risk of surgical complications, such as anastomotic leaks or vascular injuries.
5. *Improved Patient Outcomes:* Reduced rates of complications, shorter hospital stays, and improved aesthetic and functional outcomes are all linked to the effective use of ICG in onco-surgical procedures.
6. *Minimally Invasive Integration:* ICG imaging is easily adaptable to minimally invasive techniques such as laparoscopy and robotic-assisted surgery.
Challenges and Limitations
Despite its significant advantages, the application of ICG is not without challenges:
*Variability in Uptake and Interpretation:* There can be considerable variability in the uptake of ICG among different tumors and patients. Factors such as tumor type, vascularity, and the timing of administration can impact the visualization quality, leading to potential false positives or negatives.
*Lack of Standard Protocols:* Current practices regarding ICG administration vary widely across institutions and surgical specialties. The development of standardized protocols is essential for optimizing its effectiveness and ensuring consistency in clinical practice.
*Training Requirements:* Implementing ICG effectively in surgical oncology requires specialized training to interpret the fluorescence and integrate it into the surgical workflow. This can be a barrier to its widespread adoption.
Future Perspectives
The potential for ICG in cancer surgery continues to expand.
- *Targeted Delivery Systems:* Ongoing research aims to enhance the specificity of ICG through the development of conjugates that can specifically target tumor cells or their microenvironment. This advancement could improve the visualization of malignancies that currently exhibit weak fluorescence.
- *Integration with Advanced Imaging Systems:* As imaging technology evolves, the integration of ICG with advanced intraoperative imaging modalities, such as fluorescence imaging systems, will likely become more prevalent. This enhancement promises to improve identification and differentiation of cancerous tissues.
- *Clinical Trials and Evaluation:* Continued exploration of ICG usage in various cancer types through clinical trials will help establish its effectiveness across broader applications, solidifying its role in the surgical management of cancers.
Conclusion
Indocyanine Green (ICG) fluorescence imaging has become a cornerstone in advancing surgical oncology. By facilitating real-time, high-precision visualization of tumors, lymphatics, and vascular structures, ICG empowers surgeons to achieve superior outcomes for cancer patients. While challenges such as limited depth penetration and high equipment costs remain, ongoing technological advancements and research are expected to overcome these hurdles. With the continued evolution of ICG-guided techniques, the future of surgical oncology holds the promise of even greater precision, minimally invasive approaches, and improved patient care, ultimately transforming the landscape of cancer treatment.
References
1. *Tummers, Q., & Ntziachristos, V.* (2018). "Fluorescence-guided surgery: a new concept in the surgical theater." Nature Reviews Chemistry, 2(4), 1-11.
2. *Santos, T.M., et al.* (2020). "Indocyanine green for intraoperative visualization: An up-to-date review." Surgical Oncology, 34(3), 123-132.
3. *Huber, G. et al.* (2019). "Intraoperative indocyanine green fluorescence for monitoring tissue perfusion: A review." International Journal of Surgery, 67, 58-66.
4. *Kuo, W.T. et al.* (2017). "Indocyanine green fluorescence imaging in reconstructive surgery: A review." American Journal of Surgery, 213(1), 217-226.
5. *Koc, A. & Koc, A. S.* (2021). "Techniques and applications of indocyanine green in laparoscopic surgery for biliary pathology." The British Journal of Surgery, 108(6), 1-11.
6. *Matsumoto, K. et al.* (2020). "Fluorescence-guided surgery in gynecologic oncology: Potential clinical applications." Gynecologic Oncology, 158(3), 756-762.